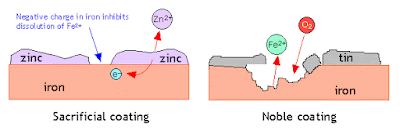
Q: Why tin Plated iron rusted rapidly when tin layer is broken while zinc coated iron does not rust even after the coating surface is broken?
Ans: The oxidation potential of Tin (Sn) is less than that of Zinc (Zn). That is why after when the surface is broken, Tin cannot provide electrons effectively while Zinc can provide electrons to iron not allowing it to be oxidized. Tin protects the iron only as long as its protective layer remains intact with iron while Zn protects the iron against corrosion even after the coating surface is broken.









