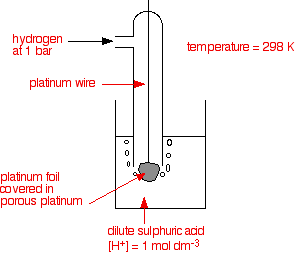
Q: Why we use Platinum foil in Standard Hydrogen Electrode?
Ans: Because hydrogen
adsorbs on Platinum foil which facilitates the equilibrium between H+
from HCl solution and adsorbed H2
2H+(aq)<========> H2 (g)
Secondly,
Platinum being noble metal is un-reactive and does not react with HCl.Further Studies CLICK HERE









